CLIA-Certified Tests
As a CLIA-certified laboratory, we adhere to the highest regulatory and quality standards, ensuring reliable and reproducible results. By integrating advanced biomarker technologies with clinical expertise, we provide specialized diagnostic and clinical development services that support therapeutic drug and medical device programs. Our capabilities span biomarker discovery, validation, and analysis, lab-developed test (LDT) development, companion diagnostics, and clinical trial support. These services play a critical role in patient stratification and selection, empowering precision medicine and enabling more effective therapeutic interventions.
Comprehensive Diagnostic Services
💠Biomarker Discovery & Validation
Leverage genomics, proteomics, and metabolomics to identify and validate novel biomarkers that improve disease diagnosis, prognosis, treatment response, and patient stratification in clinical development.
💠Multi-Platform Biomarker Analysis
Access advanced biomarker analysis through CLIA-certified platforms, including flow cytometry, multiplex assays, and other high-throughput technologies for accurate patient selection and response monitoring.
💠Lab Developed Tests (LDTs)
Develop and validate LDTs for a wide range of clinical applications, ensuring accuracy, reproducibility, and clinical relevance in diagnostic and therapeutic decision-making.
💠Companion Diagnostics (cDx)
Collaborate with biopharma partners to design and commercialize cDx tests, including IHC-based assays, to guide patient selection and optimize therapeutic outcomes.
💠Sponsored Testing Programs
Design and implement sponsored testing programs that support drug development, patient selection, and clinical decision-making for targeted and effective treatment strategies.
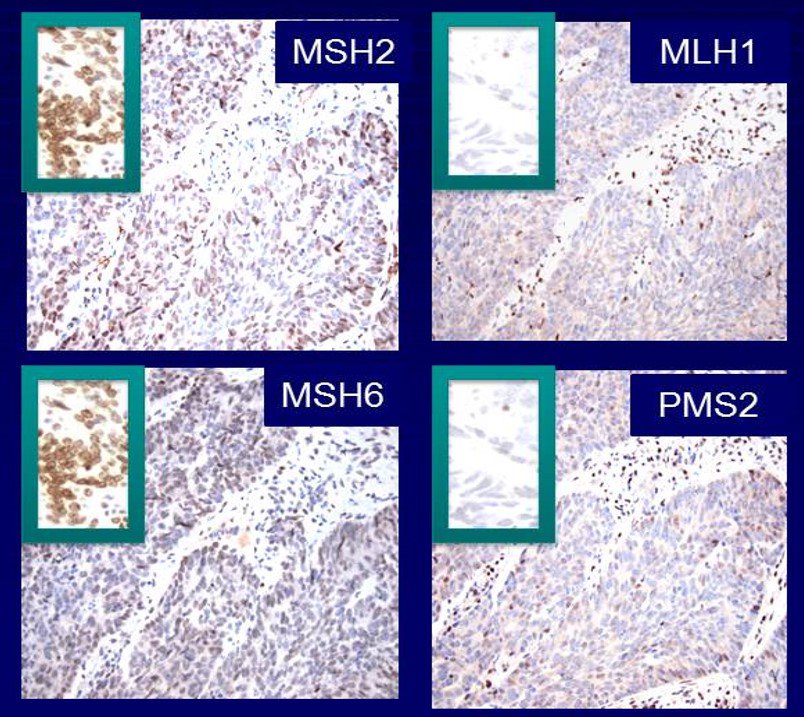
CLIA-Certified Biomarker Testing – Mismatch Repair (MMR) IHC Assay:
Representative immunohistochemistry (IHC) images showing staining for mismatch repair (MMR) proteins MSH2, MLH1, MSH6, and PMS2 in tumor tissue. Loss of nuclear staining in tumor cells, with intact staining in internal controls (insets), indicates deficient mismatch repair (dMMR) status. This biomarker assay is widely used in CLIA-certified diagnostics to evaluate microsatellite instability (MSI), guide patient stratification, and support precision oncology. By integrating such assays into companion diagnostics and clinical testing programs, we help enable more effective therapeutic decision-making, including patient selection for immunotherapy.