PhosphoFlow Analysis
PhosphoFlow enables the detection of phosphorylated proteins at the single-cell level using fluorescently labeled antibodies. This powerful approach allows simultaneous analysis of multiple phosphoproteins across distinct immune cell subsets, providing mechanistic insights into cellular signaling pathways. Compared to traditional methods like Western blotting, PhosphoFlow offers superior sensitivity, specificity, and the ability to resolve cellular heterogeneity.
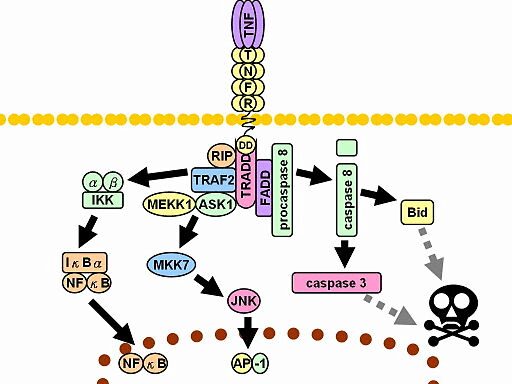
Diagram of TNF receptor signaling showing phosphorylation-driven pathways that activate NF-κB and AP-1, or trigger apoptosis via caspase signaling. These key phosphoprotein events can be measured using PhosphoFlow cytometric analysis.
How the Assay Works
✅ Treated cells or blood/tissue samples are stained with viability dye and cell surface markers.
✅ Cells are fixed and permeabilized.
✅ Intracellular phosphoproteins are labeled with fluorophore-conjugated antibodies.
✅ Samples are acquired on a flow cytometer for multiparametric analysis.
✅ Minimal volume required
✅ Broad compatibility: cultured cells, PBMCs, whole blood, or isolated tissue cells (e.g., skin cells)
Assay Benefits
Service Features
✅Superior specificity: resolves signaling heterogeneity not detectable by bulk assays
✅Flexibility: adaptable to compound screening and custom projects
✅Simultaneous detection: profile multiple phosphoproteins in diverse cell subsets
✅Single-cell resolution: quantitative, mechanistic insights into signaling dynamics
✅Standardized platform: 96-well CytoFlex S system with validated reagents
✅High-throughput capability (96-well format)
✅Multiplex quantitation of phosphoproteins across multiple cell types
✅State-of-the-art platforms: CytoFlex S flow cytometer
✅Extensive phosphoprotein antibody inventory
✅Expert analysis with 20+ years of experience
✅Timely results: 1–4 weeks depending on project scope
Example Data (a)
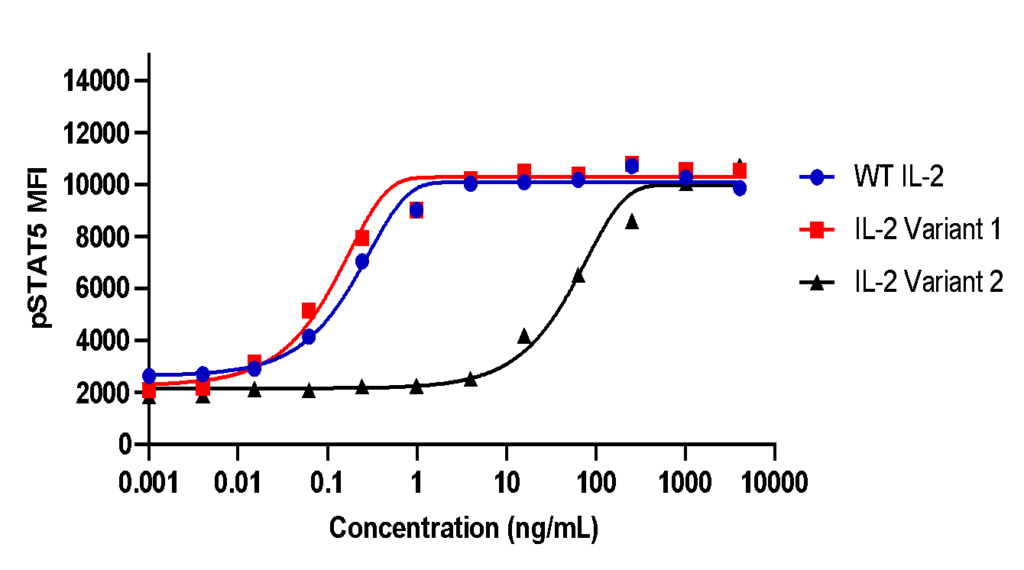
Example (a) The effect of IL-2 treatment on STAT5 phosphorylation in Tregs. Human PBMCs were treated with varying concentrations of wildtype (WT), Variant 1 or Variant 2 recombinant IL-2 for 2 h. The cells were then stained with appropriate fluorophore-conjugated antibodies and acquired on the Cytoflex S cytometer. The mean fluorescence intensity (MFI) of phosphorylated STAT5 (pSTAT5) was determined at each concentration.
Example Data (b)
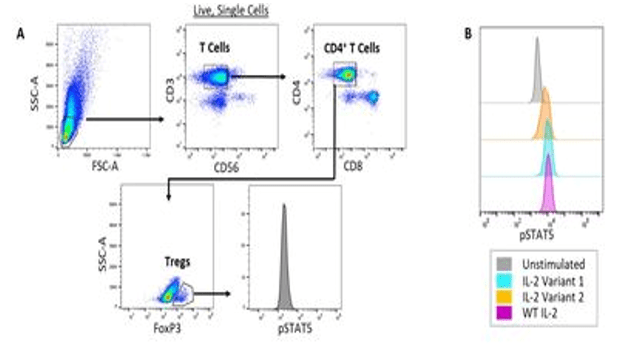
Example (b) Multidimensional analyses with phospho-specific flow cytometry. (A) Gating strategy to identify Tregs and examine STAT5 phosphorylation in a human PBMC sample. Human PBMCs were treated with 60 ng/mL of wildtype (WT), Variant 1 or Variant 2 recombinant IL-2. The cells were then stained with fluorescently-conjugated antibodies against CD3, CD56, CD4, CD8, FoxP3 and phosphorylated STAT5 (pSTAT5). (B) The level of STAT5 phosphorylation in Tregs in response to a fixed concentration of different IL-2 variants was analyzed.
Example Data (c)
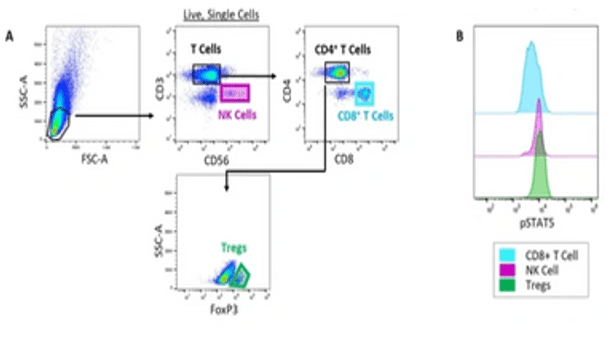
Example (c) Evaluation of pSTAT5 in multiple cell subsets following IL-2 treatment. (A) Gating strategy to identify Natural Killer (NK) cells, CD8+ T cells and Tregs in a PBMC sample. Normal human PBMCs were treated with 60 ng/mL of wildtype (WT) recombinant IL-2. The cells were then stained with fluorescently-conjugated antibodies against CD3, CD56, CD4, CD8, FoxP3 and phosphorylated STAT5 (pSTAT5). (B) The level of STAT5 phosphorylation in Natural Killer (NK) cells, CD8+ T cells and Tregs in response to WT IL-2 was analyzed.