Monocyte, Macrophage & Dendritic Cells Assay
Human, Monocyte, Macrophage & Dendritic Cell Assays
Monocytes are key immune cells originating from hematopoietic stem cells in the bone marrow. Circulating in the blood, they migrate into tissues during infection or injury and differentiate into inflammatory macrophages that drive immune defense.
Dendritic cells (DCs) act as professional antigen-presenting cells, bridging innate and adaptive immunity by activating T cells and shaping immune responses. Together, these cells are central to infection control, tumor surveillance, and immunotherapy research.
Our services include:
Target expression profiling of subsets (resting vs. activated)
Screening for modulation of M1/M2/MDSC phenotypes and differentiation
Tumor-conditioned response assays (phenotype and cytokine/chemokine production)
Antigen presentation and immune activation studies
Antibody-dependent cellular phagocytosis (ADCP)
Monocyte Activation Test (MAT) for pyrogen detection in pharmaceutical products
Monocyte Activation Test (MAT) Assay
Pyrogenic substances in pharmaceuticals can trigger severe reactions. To ensure patient safety, regulatory agencies require pyrogen testing to confirm that contaminant levels remain below acceptable thresholds.
Principle of MAT Assay
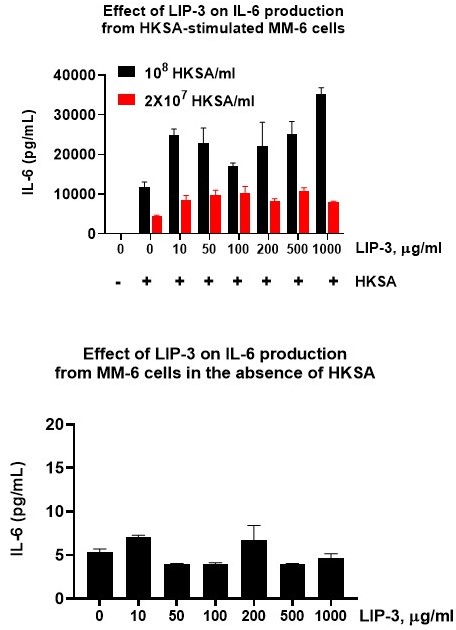
The monocyte activation test (MAT) is the in vitro alternative to the rabbit pyrogen test (RPT). Using human monocytes, it detects the full range of pyrogens — both endotoxins and non-endotoxins — by measuring cytokine release such as IL-6. These cytokines are then quantified using immunoassays.
Axela MAT Assay
We employ cryopreserved human PBMCs or Mono-Mac-6 (MM6) monocyte cells as a source of monocytes. Our MAT assay is optimized for sensitivity and flexibility, enabling detection of both endotoxin and non-endotoxin pyrogens (e.g., flagellin, HKSA, R848, PAM3CSK4). Readouts include ELISA (IL-6, IL-1β), FluoroSpot, or other tailored immunoassays.
By combining regulatory compliance with advanced assay platforms, our MAT solutions ensure accurate pyrogen detection for safe and effective pharmaceutical development.
Example Data