In Vitro Toxicology
Immunotoxicity Assays & Testing
Immunotoxicity describes the harmful effects of pharmaceuticals or other xenobiotics on the immune system. Disruption can result in immunosuppression, hypersensitivity, or overactivation—raising risks of infection, autoimmune disease, or allergies. Assessing immune safety is therefore essential in drug development.
While traditionally studied in animal models, in vitro testing offers clear advantages: mechanistic insights, better cross-species extrapolation, and reduced reliance on animals. Following FDA guidance, Axela applies standardized immunology and flow cytometry protocols to provide reliable data that support drug discovery and development.
Our testing portfolio covers both humoral and cellular immunity, including:
💠Humoral Immunity
Quantification of serum immunoglobulins and albumin-to-globulin (A/G) ratios
Isotype distribution of IgM, IgG, IgA, and IgE
Cytokine immunoassays
Hemolytic complement activity and component analysis
Auto-antibody screening and immunoglobulin deposition
💠Cellular Immunity
Flow cytometric profiling of B, T, and NK cells and subsets
Mitogenic stimulation assays for B and T cell function
Macrophage and neutrophil functional assays
Mixed Lymphocyte Reaction (MLR)
Dendritic cell maturation studies
Immunopathology evaluations
Leukocyte counts and differential analysis
Hematotoxicity Assays
Hematotoxicity describes the adverse effects of drugs on blood-forming systems, including hematopoietic stem cells (HSCs). Since HSCs replenish multiple blood cell lineages, disruption can impair erythroid, myeloid, or megakaryocyte development—often observed as side effects in cancer treatment. Evaluating hematotoxicity early in drug development is therefore essential for predicting safety and guiding clinical use.
Colony Forming Cell (CFC) Assay
The CFC assay measures the ability of hematopoietic progenitors to proliferate and differentiate into colonies in semi-solid culture media. Colony morphology provides a quantitative measure of myelotoxic potential.
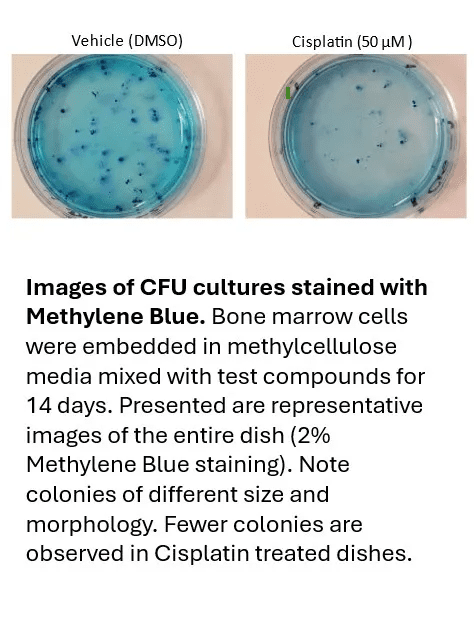
💠Advantages
Clinically predictive information supporting better planning and reduced animal use
Use of primary hematopoietic cells from multiple species (human, primate, mouse, rat, dog)
Evaluation of proliferation and differentiation of erythroid (BFU-E) and myeloid (CFU-GM) progenitors
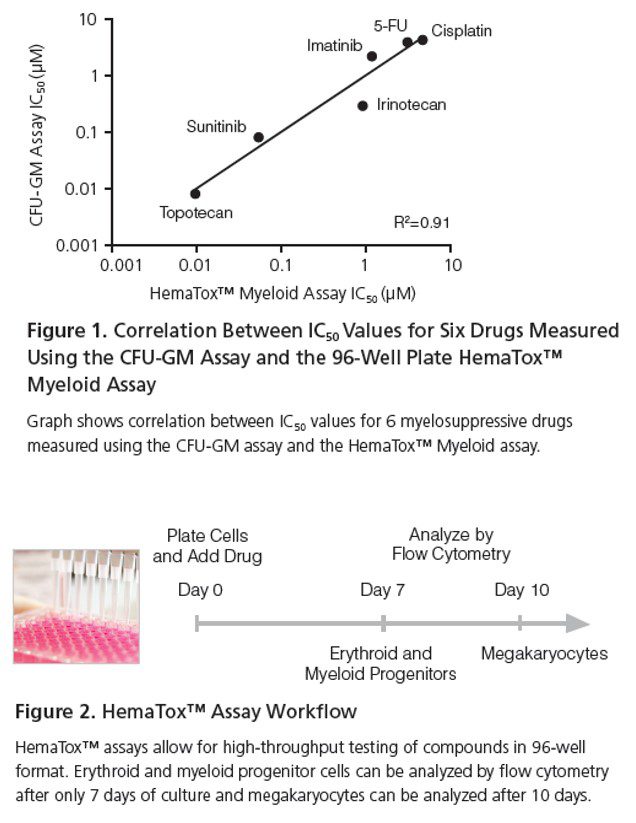
HemaTox™ Liquid Medium-Based Assay
The HemaTox™ platform enables high-throughput analysis of hematopoietic progenitors in a 96-well format, allowing faster evaluation of drug-induced hematotoxicity.
💠Advantages
Assessment of erythroid, myeloid, and megakaryocyte progenitors in <10 days
High-throughput capacity for compound screening
Strong correlation with CFC assay toxicity levels
Flexibility to test compounds at multiple time points to observe effects at different differentiation stages
Enhanced sensitivity for detecting antiproliferative effects
Genotoxicity Assay
Genotoxicity refers to the ability of chemicals or drugs to damage DNA, either directly or indirectly, leading to mutations, chromosomal instability, or heritable defects. Because genotoxic compounds may increase cancer risk, regulatory guidelines require early evaluation of genotoxicity during drug development to minimize late-stage failures.
💠Axela provides a comprehensive panel of genotoxicity assays, including:
Ames Test (OECD471): Standard assay for mutagenic damage in bacterial reverse gene mutation.
COMET Assay: Single-cell gel electrophoresis (“comet” appearance of damaged DNA) for detecting DNA strand breaks in individual cells.
In vitro Micronucleus Test (MNT, OECD487): Measures chromosomal damage through micronuclei formation.
In vitro Chromosomal Aberration Test (OECD483): Detects structural changes in chromosomes.
Immunofluorescence staining of pH3: Marker for G2/M phase progression.
Immunofluorescence staining of γH2AX: Marker of double-strand DNA breaks.
Additional customized cell-based assays available upon request.
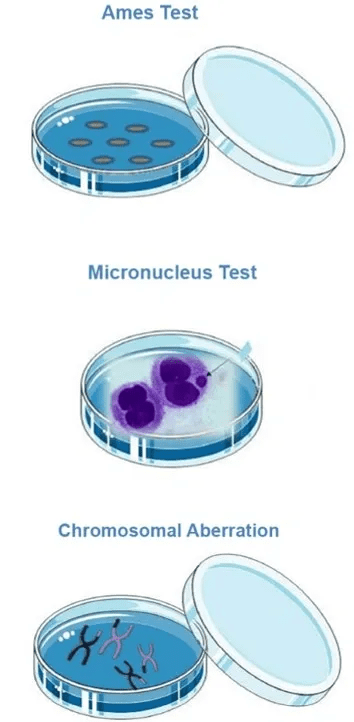
The Ames test detects mutagenic potential in bacterial cells, the Micronucleus test evaluates chromosomal damage by identifying micronuclei in dividing cells, and the Chromosomal Aberration assay identifies structural changes in chromosomes. Together, these assays provide complementary insights into a compound’s potential to induce genetic damage.